
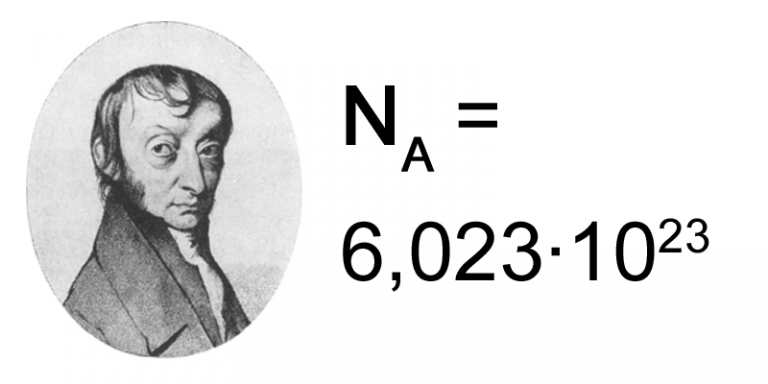
I tried googling for "standard carbon isotope ratio" but didn't find anything definitive. With the ratio of each, the average value comes out to 12.0107.īut where does that number come from? Is it by definition? Is it by convention? Is it a practical number? Where do the error bars come from? For instance, I would expect to see different isotope distributions at different geographic locations (or what about the moon, asteroids, Jupiter, etc.). Carbon disulfide: CS2: 76.13: Carbon Disulfide: CS2: 76.13 g/mol: Carbon Disulphide: 76.13: Carbon monoxide: CO. Molar mass of 465 common chemical compounds Molecular.
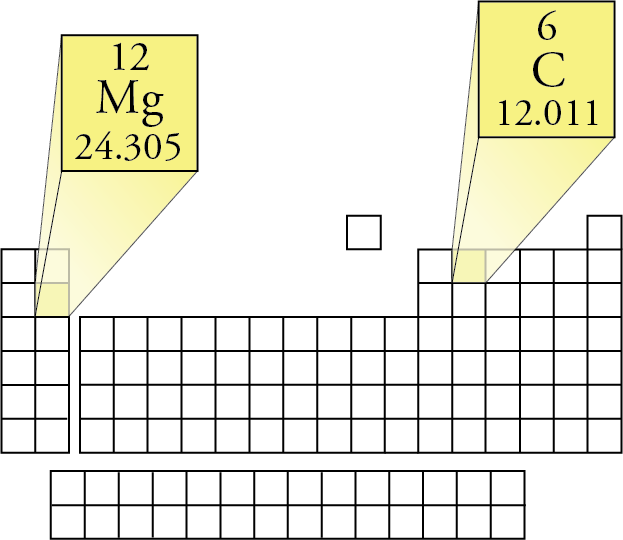
This example shows how the atomic weight of an element differs from the atomic masses of its isotopes. Table of data giving the molar mass of many common substances used in process industries. When I asked on a forum why this isn't precisely 12, I was told that typically carbon is a mixture of isotopes which have relative atomic masses of 12, 13 and 14. Carbon is a mixture of two isotopes: 12C and 13C. By definition, carbon-12 has a molar mass of 12 g/mol. The molar masses are 44.01 g/mol and 78.04 g/mol respectively. One mole of carbon dioxide molecules has a mass of 44.01 g, while one mole of sodium sulfide formula unit has a mass of 78.04 g. The molar mass of any compound is the mass in grams of one mole of that compound. For hydrogen, we multiply the molar mass of 1.00794 by 4. Different elements have a different atomic mass, and as collections of single atoms, molecules have a definite molar mass, measured with the unit mole (6.02 × 10 23 individual molecules, Avogadro's constant). The molecular mass of carbon dioxide is 44.01 amu. However, when I go to websites such as or and I ask what is the molar weight of Carbon, I get answers such as: Problem 3: Caffeine has the following percent composition: carbon 49.48, hydrogen 5.19, oxygen 16.48 and nitrogen 28.85. For carbon, we multiply its molar mass of 12.0107 grams per mol by two because we have two carbon atoms. The mole is defined as the amount of a chemical substance which contains as many representative particles, e.g., atoms, molecules, ions, electrons, or photons, as there are atoms in 12 grams of carbon-12 (12C), the isotope of carbon with relative atomic mass 12 (from Wikipedia). The atomic weight of an element is the weighted average of the atomic masses of the different isotopes of an element.


 0 kommentar(er)
0 kommentar(er)
